- Acne
- Alzheimers
- Anti Allergy
- Anti Biotic
- Anti Cancer
- Anti Cougulant
- Anti Depressant
- Anti Diabetics
- Anti Parkinson
- Anti Prostate Cancer
- Anti Pyscotic
- Anti Sleep
- Birth Control
- Blood Pressure
- Bodybuilding
- Breast cancer
- Constipation
- Contrakit
- Diarreha
- EFIL 5 MG-1
- Erectile Dysfunction
- Eye Care
- Fungal Infection
- growth Hormones
- Hair Care
- Heart Attack
- High Blood Pressure
- High Cholestrol
- Inhalers
- Insomnia
- Men's Health
- Migrane
- MultiVitamins
- Muscle Relaxant
- Nausea
- Painkillers
- Parkinson
- Skincare
- Steroids
- Tablet
- Uncategorized
- Weight Loss
- Women's Health
$48.00 – $132.48Price range: $48.00 through $132.48

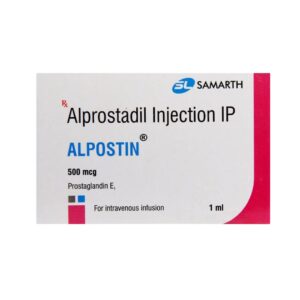
Alpostin Alprostadil 500mcg
$501.22 – $1,474.19Price range: $501.22 through $1,474.19
Alpostin Alprostadil 500mcg — Alpostin 500mcg Injection — Alpostin 500mcg Injection – Overview
🔥 Buy more save more!
Buy from 2 to 5 items and get 5% OFF
on each productBuy from 6 to 10 items and get 10% OFF
on each productAlpostin 500mcg Injection – Overview
What is Alpostin 500mcg Injection?
Alpostin 500mcg Injection is a medication used to treat patent ductus arteriosus (PDA), a heart defect that occurs in newborns. PDA is a condition where a blood vessel in the heart does not close after birth, affecting normal blood circulation. Alpostin helps improve blood flow in these babies, aiding in the treatment of this heart defect.
How is Alpostin 500mcg Injection Given?
Alpostin 500mcg Injection is administered as an injection by a qualified doctor or nurse, under strict medical supervision. It is crucial that this medication is not used outside of a healthcare setting, ensuring the baby receives the appropriate care and monitoring during its use.
Possible Side Effects:
While Alpostin 500mcg Injection is effective, some common side effects may occur. These include:
- Apnea (interrupted breathing): This is a temporary condition, particularly in newborns.
- Transient Pyrexia (Fever): A short-term increase in body temperature is common.
If your baby experiences any of these side effects, or other unexpected reactions, it’s important to inform the doctor immediately.
Precautions & Allergic Reactions:
Before administering Alpostin, your doctor will perform tests to ensure that your baby needs the medication.
However, if your baby shows any signs of allergic reactions to this medicine, such as:
- Wheezing or difficulty breathing
- Swelling of the face, hands, or other areas
- Itchy rash
- Redness of the skin
Please do not use this medicine and consult your doctor right away. If you have any concerns or doubts regarding its use, feel free to reach out to your healthcare provider for clarification and guidance.
Note:
It is important to remember that Alpostin 500mcg Injection is administered under medical supervision and should only be used as directed by a healthcare professional to ensure the safety and well-being of your baby. Always consult your doctor if you have any concerns or questions about the treatment.
| Quantity | 3 Injection, 6 Injection, 9 Injection |
|---|
5.00
5 reviews for Alpostin Alprostadil 500mcg
Only logged in customers who have purchased this product may leave a review.
Buy 1 Get 1 Free
Unbetable Quality
Recent Products

Use Coupon Code
Z24U
Recent Products
Our Partners





Careprsot Eye Drop 0.03
CEFLOX EYE DROP
Omnacortil 10Mg
Super Lash Ophthalmic Solution
By using this website you agree to our Privacy Policy.
You may add any content here from XStore Control Panel->Sales booster->Request a quote->Ask a question notification
At sem a enim eu vulputate nullam convallis Iaculis vitae odio faucibus adipiscing urna.












Jonathan Reed –
Genuine medicine, worked exactly as expected.
Aarav Mehta –
Noticed improvement within a few days.
Eli Cohen –
Fast delivery and properly sealed packaging.
Oliver Hughes –
Safe and effective, no side effects for me.
Liam Thompson –
Good price for an original product.